Expert: Time, contaminants can affect substrate surface, reduce adhesive bond strength at molecular level
By onMarket Trends | Repair Operations | Technology
It might be far easier than you’d expect to sully a substrate a technician has painstakingly prepped for adhesive — and thereby weaken the subsequent bond, an expert advised during a Composites World webinar last week.
BTG Labs chief scientist Giles Dillingham’s presentation Oct. 17 was tailored to manufacturers using substances like carbon-fiber reinforced polymers and other composites, but he said the same basic principles would apply to aftermarket auto body repair and the bonding of steel and aluminum.
“They absolutely exist with any surface,” Dillingham said.
The same laws of chemistry apply, after all. Abrasion (e.g., a technician sanding a surface), chemical treatment or another surface prep process breaks the bonds of a substrate’s outer molecules during the surface prep process, boosting the energy of the substrate and rendering those treated exterior molecules highly reactive. (Think “sticky.”)
The molecules in the adhesive, meanwhile, are looking to react with something with high surface energy. They find the broken bonds of the substrate’s molecules, lock together with them and settle into the lowest-energy configuration possible. After some curing, voila, you’ve got a bonded joint.
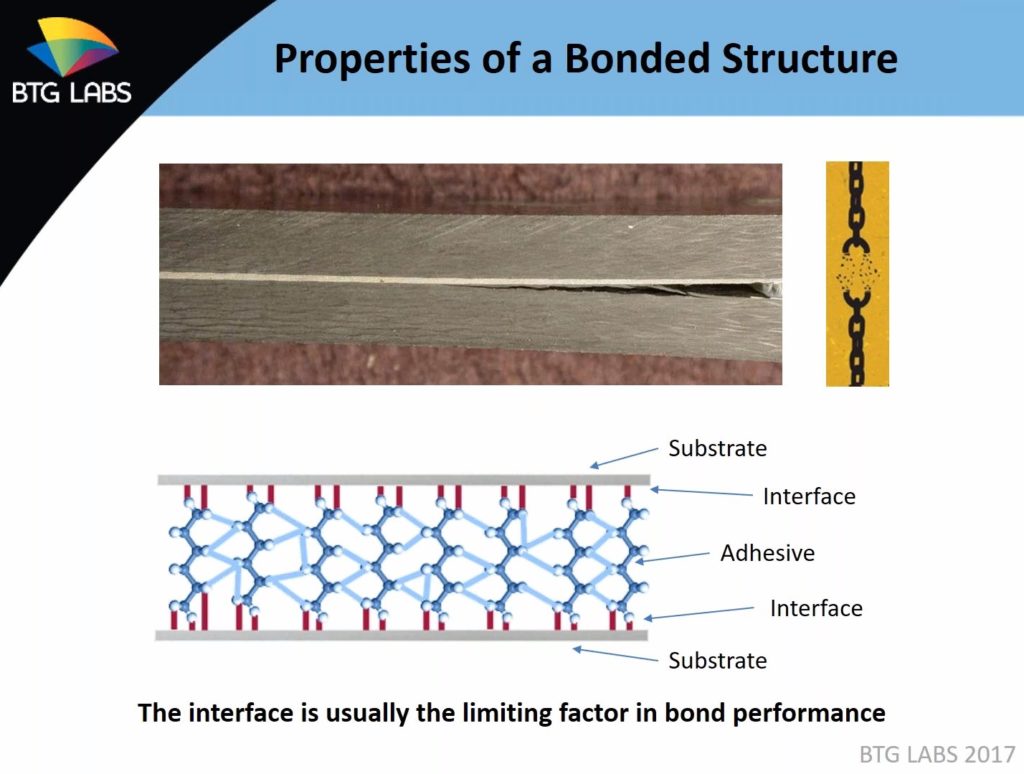
While a technician or factory worker might apply a thick bead of adhesive, the actual interface — the bond between the exterior molecules of the adhesive and the exterior molecules of the substrate — is extremely thin, as little as six molecules thick, according to Dillingham. By comparison, a fingerprint from a freshly washed hand’s oils is 1,000 molecules thick, and the organic molecules deposited by your breath are 40-100 molecules thick.
But that 6-molecule-thick connection (the red lines in the image above) is also the most important part of the bonding process, as it’s what’s actually holding the joint together. Dillingham said all bonds, whether aluminum, steel or carbon fiber, come down to “a few molecular layers.” (Similarly thin interfaces also apply for automotive refinishing.)
“That’s the heart of the bond there,” Dillingham said. “… That interface is usually the limiting factor in bond performance.”
Learn about joining during Repairer Driven Education
Learn about joining during SEMA with Toby Chess of Kent Automotive at “Adhesive Joining in Modern Repairs” and Dave Gruskos of Reliable Automotive Equipment with “Get Attached to Following Procedures: A Comprehensive Guide to OEM Joining.” The sessions are part of the Society of Collision Repair Specialists Repairer Driven Education Series Oct. 30-Nov. 3. Register here for individual classes or the series pass package deal, which includes the entire week of classes, all three parts of the OEM Summit and the Nov. 2 Sky Villa afterparty.
Dillingham said audience members had probably encountered a bond which failed despite the usual procedures being followed. The interface was likely to blame. Optimizing that connection is crucial to avoid such “nasty surprises,” Dillingham said.
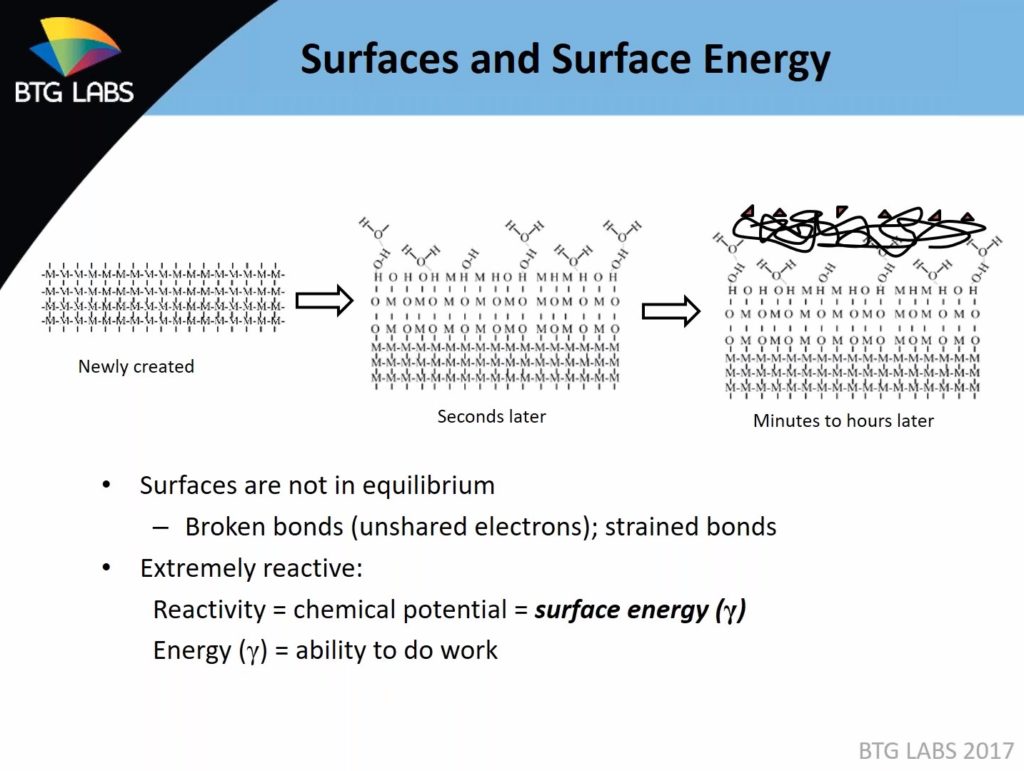
Time proves a major factor in the integrity of the interface, according to Dillingham. Science dictates that such a surface prepped for an adhesive will within a matter of seconds begin to react with other atoms floating about the shop and form bonds with those molecules — reducing the reactivity of the underlying substrate. Touch or breathe on a prepared substrate, and you’re contributing to the problem.
Eventually, a thin layer of less reactive oxide, water and “anything that’s floating around” coats the surface — and the adhesive ends up sticking more to that instead of the intended substrate, according to Dillingham.
The joint might look fine to the naked eye, but it’s actually far weaker than it should be at the microscopic level, as there’s less surface energy for the adhesive to use for bonds. The higher the surface energy, the higher the bond strength, Dillingham said.
So the moral of the story (besides following whatever the OEM says): Don’t let a prepared part sit too long, keep your shop clean, and keep your grubby mitts off the surface being bonded. Dillingham noted that an aircraft or automobile in service for a while would have “a very dirty surface” around the area to be bonded, potentially making the task harder than would be the case with a new part under more controlled conditions like a factory.
“One has to take extreme care in prepping that surface to a molecular level of cleanliness,” Dillingham said of a repair environment.
Dillingham said it would be nice if adhesive manufacturers offered more surface preparation guidelines and didn’t focus their efforts solely on the adhesive. “They’re typically not experts on surface prep,” he said.
BTG Labs does sell a hand tool to ensure a substrate is adequately reactive to achieve the desired bond strength, which seems like a useful way of checking the quality of a technician’s surface preparation and verifying that a prepared part’s reactivity wasn’t overly compromised by being left out in the open too long.
The device, which resembles a boroscope, sprays a microdroplet of ultrapure water on the substrate and measures the angle between the bottom of the drop and the surface. The more the water beads rather than spreads, the higher the contact angle and the more likely the bond itself will fail before the substrate, Dillingham said. He said a rule of thumb holds that an angle of 30-40 degrees or less is a good surface. If you picked up a composite substrate sitting on a bench for a while, it’d have an angle of around 60-70 degrees.
“You want somewhere around a 30-degree contact angle,” he said.
The tool can run $15,000-$25,000 (though it can be leased), and the company’s focus has been aerospace and automotive manufacturing rather than the automotive aftermarket. However, it doesn’t seem like a stretch to see this sort of quality control measure being required by luxury European OEMs in the near future, depending on the rate of composite adoption. Technically, since it’s all the same bonding science, an extremely meticulous paint shop could also be checking the quality of their prepper’s work prior to refinishing the substrate.
More information:
“Development and control of surface treatments for adhesive bonding of composites”
Composites World, Oct. 17, 2017
Images:
Water molecules in the air over time can contaminate a surface prepared by a body shop to receive adhesive. (olegback/iStock)
Abrasion, chemical treatment or another surface prep process breaks the bonds of a substrate’s outer molecules during the surface prep process, boosting the energy of the substrate and rendering those treated exterior molecules highly reactive. The molecules in the adhesive, meanwhile, are looking to react with something with high surface energy. They find the broken bonds of the substrate’s molecules, lock together with them and settle into the lowest-energy configuration possible. After some curing, voila, you’ve got a bonded joint. (Provided by BTG Labs)
Time proves a major factor in the integrity of a bond interface, according to BTG Labs. Science dictates that such a surface prepped for an adhesive will within a matter of seconds begin to react with other atoms floating about the shop and form bonds with those molecules — reducing the reactivity of the underlying substrate. Touch or breathe on a prepared substrate, and you’re contributing to the problem. Eventually, a thin layer of less reactive oxide, water and “anything that’s floating around” coats the surface — and the adhesive ends up sticking more to that instead of the intended substrate, according to BTG Labs chief scientist Giles Dillingham. (Provided by BTG Labs)